Indication & Important Safety Info
Indications for PENMENVY, BEXSERO, and MENVEO
Important Safety Information for PENMENVY, BEXSERO, and MENVEO
Indications for PENMENVY, BEXSERO, and MENVEO
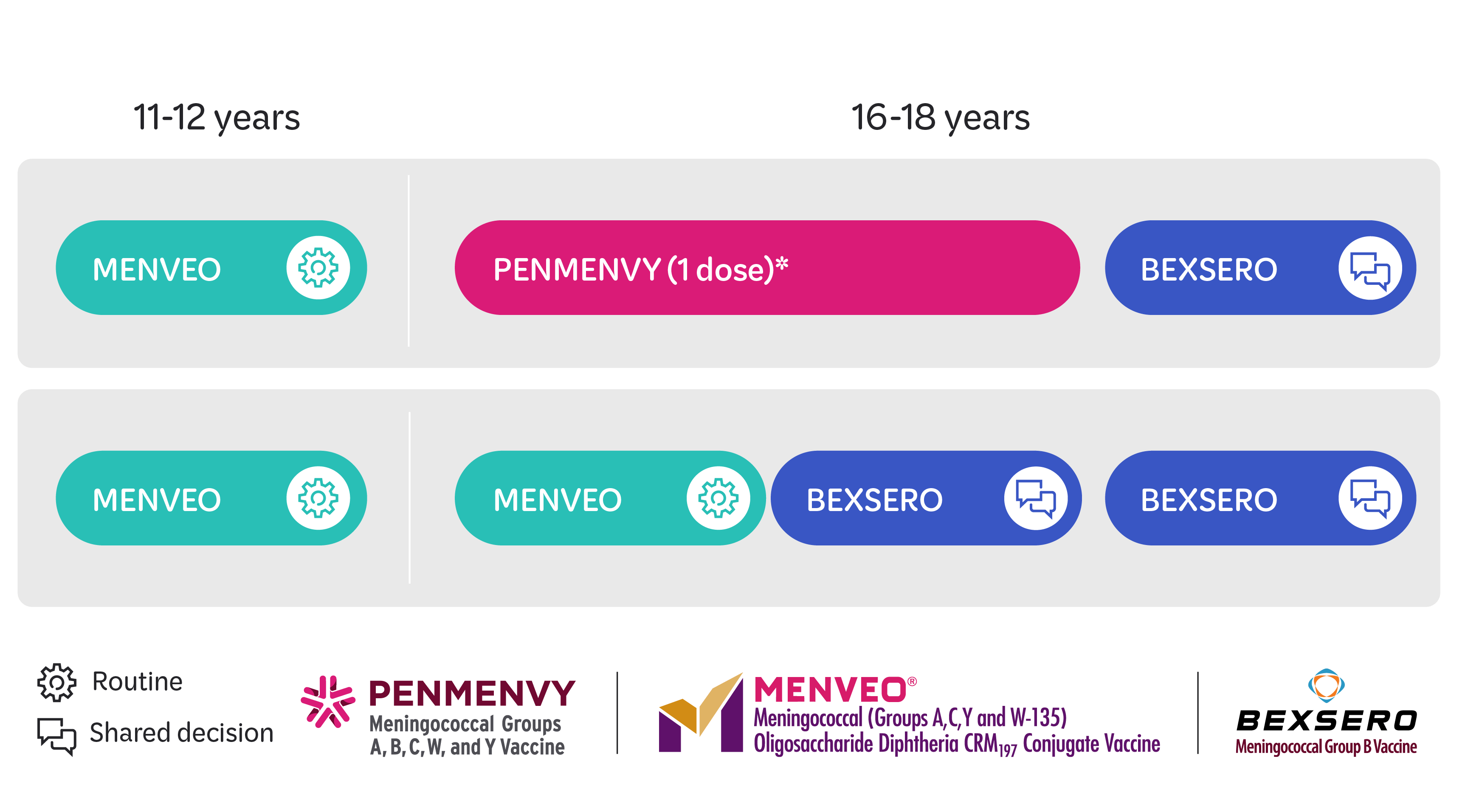
PENMENVY is a vaccine indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroups A, B, C, W, and Y in individuals 10 through 25 years of age.
BEXSERO is a vaccine indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. BEXSERO is approved for use in individuals aged 10 through 25 years.
MENVEO is a vaccine indicated for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135 in individuals 2 months through 55 years of age. MENVEO does not prevent N. meningitidis serogroup B infections.
Important Safety Information for PENMENVY, BEXSERO, and MENVEO
- Do not administer PENMENVY to individuals with a severe allergic reaction (e.g., anaphylaxis) to a previous dose of PENMENVY, to any component of this vaccine, or to any other diphtheria toxoid-containing vaccine
- Do not administer BEXSERO to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of BEXSERO or after a previous dose of BEXSERO
- Do not administer MENVEO to individuals with a severe allergic reaction (e.g., anaphylaxis) to a previous dose of MENVEO, to any component of MENVEO, or to any other diphtheria toxoid-containing vaccine
- Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of PENMENVY, BEXSERO, or MENVEO
- For BEXSERO, the tip cap of the prefilled syringe may or may not be made with natural rubber latex. Natural rubber latex may cause allergic reactions. Please check the carton
- Syncope (fainting) has occurred in association with administration of PENMENVY, BEXSERO, or MENVEO. Ensure procedures are in place to avoid injury from falling associated with syncope
- PENMENVY, BEXSERO, or MENVEO may not protect all vaccine recipients, and PENMENVY or BEXSERO may not protect against all meningococcal serogroup B strains
- Immunocompromised persons and some individuals receiving immunosuppressant therapy may have reduced immune responses to PENMENVY, BEXSERO, or MENVEO
- Individuals with certain complement deficiencies and individuals receiving treatment that inhibits terminal complement activation (for example, eculizumab) are at increased risk for invasive disease caused by N. meningitidis serogroups A, B, C, W, and Y, even if they develop antibodies following vaccination with PENMENVY, BEXSERO, or MENVEO
- Guillain-Barré syndrome (GBS) has been reported in temporal relationship following administration of another US-licensed meningococcal quadrivalent polysaccharide conjugate vaccine. The decision to administer PENMENVY or MENVEO to individuals with a history of GBS should take into account the expected benefits and potential risks
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. A decision about when to administer MENVEO to an infant born prematurely should be based on consideration of the individual infant’s medical status and the potential benefits and possible risks of vaccination
- For PENMENVY, the most commonly reported (≥10%) solicited adverse reactions in individuals aged 10 through 25 years after Dose 1 and Dose 2, respectively, were pain at the injection site (92% and 88%), fatigue (51% and 42%), headache (42% and 36%), myalgia (15% and 12%), nausea (15% and 10%), erythema (13% and 12%), and swelling (13% and 12%). The most commonly reported (≥10%) solicited adverse reactions in MenACWY conjugate vaccine-experienced individuals aged 15 through 25 years after Dose 1 and Dose 2, respectively, were pain at the injection site (80% and 74%), headache (41% and 33%), fatigue (40% and 33%), myalgia (15% and 13%), and nausea (15% and 12%)
- For BEXSERO, the most commonly reported (≥10%) solicited adverse reactions in a Phase 3 clinical trial were pain at the injection site (87%-92%), fatigue (45%-49%), headache (37%-41%), nausea (11%-13%), erythema (10%-15%), myalgia (10%-14%), and swelling (10%-14%)
- Common solicited adverse reactions with MENVEO among children initiating vaccination: at 2 months of age and receiving the four-dose series were tenderness and erythema at injection site, irritability, sleepiness, persistent crying, change in eating habits, vomiting, and diarrhea; at 7 months through 23 months of age and receiving the two-dose series were tenderness and erythema at injection site, irritability, sleepiness, persistent crying, change in eating habits, and diarrhea; at 2 through 10 years of age who received MENVEO were injection site pain, erythema, irritability, induration, sleepiness, malaise, and headache. Common solicited adverse reactions among adolescents and adults aged 11 through 55 years who received a single dose of MENVEO were pain at the injection site, headache, myalgia, malaise, and nausea. Across all age groups, some events were severe. Similar rates of solicited adverse reactions among adolescents and adults were observed following a single booster dose
- For MENVEO, in two clinical studies, there were no notable differences in frequency and severity of solicited adverse reactions in individuals who received MENVEO 1-vial presentation compared to individuals who received the 2-vial presentation
Prescribing Information for PENMENVY (Meningococcal Groups A, B, C, W, and Y Vaccine)
Prescribing Information for BEXSERO (Meningococcal Group B Vaccine)